Hepatic Fibrosis is Common in ÃÆà ½Ãâò-Thalassaemia and is Associated with Current and Historical Iron Loading and Hepatitis C
Edward Shelton, Chia Pei Chong, Lani Shochet, Jamie heong, Sim Yee Ong, Donald K Bowden, Alexander Hodge, Virginia Knight, Ken Cheng, Sant-Rayn Pasricha and Anouk Dev
1Department of Gastroenterology and Hepatology, Monash Health, Melbourne, Australia
2Medical Therapy Unit (Thalassaemia Service), Monash Health, Melbourne, Australia
3Department of Radiology, Monash Health, Melbourne, Australia, Monash Health, Melbourne, Australia
4Melbourne School of Population and Global Health, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Australia
5MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, UK
6Centre for Inflammatory Diseases, Department of Medicine, Monash University, Melbourne, Australia
- *Corresponding Authors:
- Dr. Anouk Dev
- Department of Gastroenterology and Hepatology
Monash Health, Melbourne, Australia
Tel: +61 39594 6666
Fax: +61 39594 6250
E-mail: anouk.dev@monash.edu - Dr. Sant-Rayn Pasricha
Melbourne School of Population and Global Health Faculty of Medicine
Dentistry and Health Sciences, University of Melbourne, Australia
Tel: +44 01865 222547
Fax: +61 39594 6250
E-mail: sant-rayn.pasricha@unimelb.edu.au
Received Date: December 10, 2015; Accepted Date: January 05, 2016; Published Date: January 12, 2016
Citation: Shelton E, Chong CP, Shochet L, et al. Hepatic Fibrosis is Common in β-Thalassaemia and is Associated with Current and Historical Iron Loading and Hepatitis C. J Hep. 2016, 2:1 doi:10.21767/2471-9706.100011
Abstract
Background: Transfused thalassaemia patients are at risk of cirrhosis. Transient Elastography (TE) enables non-invasive liver stiffness measurement (LSM). The Enhanced Liver Fibrosis (ELF) score has excellent correlations with liver fibrosis. We aimed to evaluate the prevalence of and risk factors for fibrosis in a cohort of adult patients with transfusion dependent thalassaemia using LSM and ELF testing.
Methods: All adults with transfusion-dependent thalassaemia at our institution were invited to undergo LSM and ELF measurements. Concurrent and historical ferritin, hepatitis C viral load, liver ultrasound and T2* MRI data were obtained.
Findings: We evaluated 63 patients (mean age 43y, 46% male, 89.9% using deferasirox) for liver fibrosis by LSM and ELF. By LSM, 29% had fibrosis, including 11% with cirrhosis. By ELF score, 34.4% had severe fibrosis. By multiple linear regression, LSM was associated with age (P=0.002), presence of detectable hepatitis C virus (HCV) (P=0.002), and both current (P<0.012) and historical ferritin level (P=0.012) concentrations (R2=0.457). By multiple regression, ELF score was associated with age (P=0.005) and historical ferritin levels (P<0.001).
Conclusions: Hepatic fibrosis and cirrhosis are prevalent in adults with transfusion dependent thalassaemia. Historical iron overload and HCV infection mediate fibrosis risk. Most adult patients with a history of prior iron loading and/or untreated HCV should undergo screening for cirrhosis, irrespective of ferritin level.
Keywords
Thalassaemia; Fibrosis; Hepatitis C Virus; Ferritin; Transient Elastography; Enhanced Liver Fibrosis Score
Abbreviations
Transfused Haemoglobinopathy (TH); Hepatitis C Virus (HCV); Hepatocellular Carcinoma (HCC), Liver Stiffness Measurement (LSM); Transient Elastography (TE); Enhanced Liver Fibrosis (ELF); Body Mass Index (BMI); International Normalised Ratio (INR)
Introduction
Patients with transfusion-dependent haemoglobinopathies are at risk of iron overload, which can produce a range of cardiac, endocrine and hepatic complications; prevention of these complications requires a regimen of iron chelation. Until recently, the major iron chelator was desferrioxamine, a nonorally bioavailable drug with a short half-life, necessitating subcutaneous infusion over several hours, most days of the week, making adherence difficult for many patients. Recently, oral chelators have become available, greatly enhancing acceptability of regular iron chelation, and potentially improving outcomes [1].
Transfused haemoglobinopathy (TH) patients are at significant risk of liver cirrhosis due to hepatic iron loading and in many cases, transfusion related Hepatitis C virus (HCV) [2]. The complications of siderosis are a function of degree of iron load and length of time over which it occurs [3]. While the improved tolerability of oral iron chelators may increase adherence, they do not eliminate hepatic effects of siderosis if iron-induced fibrosis has occurred. This process may be irreversible even with reduction in iron load. Thus, complications from cirrhosis may become increasingly prominent in this patient group as chelation ameliorates mortality from cardiomyopathy and patients are living longer [2,4]. Early identification of hepatic fibrosis and cirrhosis in this population is crucial to facilitate implementation of regular screening for complications including hepatocellular carcinoma (HCC), which has been reported to develop at a younger age in TH patients compared to the general population [4].
Until recently, there have been limited guidelines for liver fibrosis screening in the TH population [5]. Identification of the prevalence and risk factors for hepatic fibrosis could result in a recommendation that all transfusion dependent patients, or at least those with defined risk factors, undergo regular screening. Inadequacy of potential screening tests has limited accurate estimates of the prevalence of and risk factors for hepatic fibrosis among patients with transfusion-dependent thalassaemia and conventional non-invasive methods for identifying liver fibrosis including ultrasound and liver biochemistry are insensitive for assessing the degree and progression of fibrosis [6]. Although liver biopsy is considered the gold standard method in evaluating liver fibrosis and liver iron concentration, it is invasive, associated with recognised complications, limited by sampling error and generally unacceptable to asymptomatic patients, rendering it an inappropriate screening and monitoring tool [7,8]. MRI T2*/R2 methods have been validated as appropriate measures of liver iron and have replaced biopsy in many cases; however, conventional MRI is insensitive to early fibrotic changes.
Non-invasive methods have been developed to facilitate estimation of liver fibrosis, including liver stiffness measurement (LSM) by transient elastography (TE) and serum markers such as the Enhanced Liver Fibrosis (ELF) panel. LSM has been validated in many clinical scenarios including HCV and non-alcoholic fatty liver disease (NAFLD) [9-11]. It has been studied in small cohorts of patients with β-thalassaemia and found to be predictive of fibrosis and independent of liver iron levels [12,13]. The ELF panel comprises a set of extracellular matrix proteins and is accurate in predicting significant fibrosis in conditions including alcoholic liver disease, NAFLD, HCV and primary biliary cirrhosis [14-19], although not yet for thalassaemia. The ELF score was originally validated in a large group of patients with chronic liver disease of different aetiologies [14], after which it was confirmed in a number of different patient groups [17-21]. The three markers in the ELF score reflect ongoing fibrogenesis mostly at sinusoidal levels and, as such, have a higher sensitivity and negative predictive value for NAFLD, post transplant chronic HCV and hemochromatosis, which are all characterised by initial sinusoidal fibrosis [22]. The pattern of iron deposition in thalassaemia is similar to that of hemochromatosis and therefore the ELF is a potentially useful marker of fibrosis in patients with TH [23]. More recently, the combination of serum markers with LSM has been shown to increase accuracy for the detection of fibrosis [24]. Use of these methods enables non-invasive screening of large numbers of atrisk patients for fibrosis.
We sought to evaluate the prevalence of and risk factors for fibrosis in a cohort of adult patients with transfusion dependent thalassaemia using LSM and ELF. We hypothesised that liver fibrosis would be endemic, with some patients having unsuspected cirrhosis. In addition, we hypothesised that the risk of cirrhosis would be associated with both historic and present iron loading and HCV. This data should guide recommendations for screening of patients with transfusion dependent thalassaemia for hepatic fibrosis.
Methods
We undertook a prospective study of patients with transfusion dependent thalassaemia at Monash Medical Centre, the state reference centre for inherited red cell disorders in Melbourne, Victoria, Australia. The study was approved by the institutional review board of Monash Health.
Eligibility
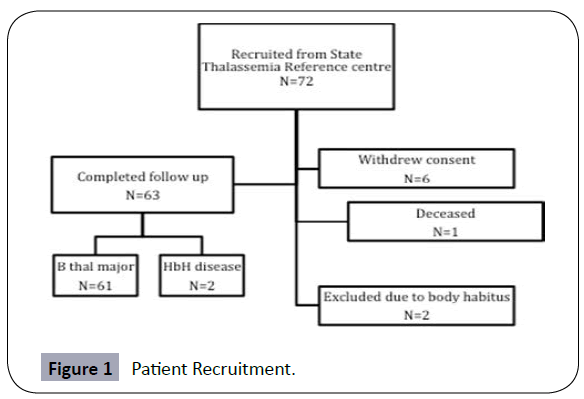
Eligibility criteria included: i) age >18 years, ii) receiving regular blood transfusions (≥10 units per year for ≥3 years) iii) clinical and genetic diagnosis of a haemoglobinopathy including β-thalassaemia major, sickle cell disease, haemoglobin H disease.
Patients were ineligible if they had one or more of the following: i) significant alcohol intake (>30g of ethanol per day) ii) Body Mass Index (BMI) > 28 [25] iii) antibodies to human immunodeficiency virus, iv) concurrent medical or psychological condition precluding compliance, and v) other causes of liver disease including Wilson’s disease, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis, alpha-1-antitrypsin deficiency or hemochromatosis. A diagnosis of chronic hepatitis B and C was permitted.
Study Procedures
The participants underwent screening blood tests including liver function tests, serology including anti-HCV antibody, HCV-RNA by real-time polymerase chain reaction, and testing to exclude other causes of liver disease including hepatitis B serology. They underwent LSM by TE (FibroScan; Echosens, Paris, France) within 30 days of enrolment. Serum was obtained for ELF panel testing. Results for liver ultrasound within 6 months and cardiac and liver MRI T2* within 1 year of TE measurement were collected. Ferritin results over the previous 15 years (first measurement of the year for 2013, 2008, 2003 and 1998) were retrieved.
Transient Elastography
Two investigators independently performed LSM (AH, VK). The patients fasted for two hours prior to the procedure. The examination was performed on the right side of the liver through the intercostal space. Only LSM results containing 10 valid measurements, with a success rate of at least 60% and an interquartile range of ≤30% were acceptable. The LSM cutoff for fibrosis was predefined according to the results of a previous study in patients with homozygous β-thalassaemia, who underwent liver biopsy and TE evaluation: [13]. In this study, the largest in this population with matched liver biopsies, LSM ≥ 13kPa had an area under the receiver operator characteristic curve (AUCROC) of 0.997 for the prediction of cirrhosis with 100% sensitivity and 95% specificity.
The Enhanced Liver Fibrosis (ELF) Score
Quantitative measurements of hyaluronic acid, amino-terminal propeptide of type III procollagen and tissue inhibitor of metalloproteinase 1 were measured on serum (Siemens ADVIA Centaur® Immunochemical analyser); an algorithm combined these to provide the ELF score. A cut-off for severe fibrosis (Ishak score 5 or 6) was defined as ≥ 9.8 units based on the manufacturer’s recommendations, developed using a range of validation clinical studies in patients with hepatic fibrosis.
T2* Magnetic Resonance Imaging
Cardiac T2* and liver T2* were assessed using validated techniques [26,27]. Patients were scanned with the Siemens 1.5- T Magnetom Avanto. Liver and cardiac iron loading assessment was evaluated with a normal liver T2* > 6.3ms and cardiac > 20ms.
Statistical Analysis
Demographic, clinical and biochemical data were summarised, and skewed data were log-transformed where necessary. Univariate linear regression analysis was performed to investigate relationships between these data and log-transformed LSM and ELF values parameters. Variables identified as associated with LSM/ ELF by univariate regression with a p<0.10 were tested in multiple regression models. Associations between risk factors and logeLSM and logeELF were evaluated by linear regression, and associations between thresholds versus normal, by logistic regression. The final multiple regression models contained independent variables, were parsimonious, and had the highest adjusted R2 value. Data were analysed using Stata 11 (Stata IC, Statacorp, College Station, Tx USA).
Results
Clinical Characteristics
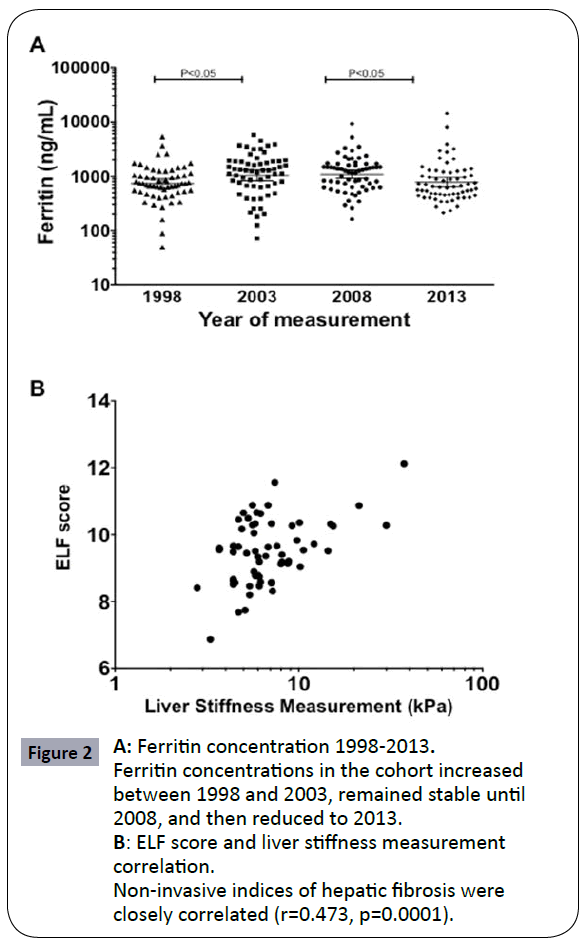
Between August–November 2012, all adult patients with transfusion dependent haemoglobinopathies at the centre were invited to participate. 72 patients were enrolled, of whom 63 completed assessment with TE (males 46%, mean age 41.3 years) (Figure 1). Table 1 summarises the clinical and laboratory characteristics of the group. 61 patients (96.8%) had β-thalassaemia major and 2 (3.2%) haemoglobin H disease; 56 patients (88.9%) were treated with deferasirox and 7 (11.1%) with desferrioxamine. HCV Ab was detectable in 54%, including 27% with detectable viral load. Mean concurrent (January 2013) ferritin was 776.5ng/uL; mean ferritin concentrations in January 1998, 2003 and 2008 were 734.5ng/uL, 1041.9ng/uL and 1091.1ng/uL respectively; a significant increase occurred between 1998-2003, and a significant reduction between 2008- 2013, coinciding with the introduction of deferasirox (Figure 2a).
| Mean [95% Confidence interval], or n/N (%) | |
|---|---|
| N | 63 |
| Sex (M:F) | 29:34 |
| Age (years)1 | 41.3 [39.2, 43.4] |
| Condition Beta-thalassaemia major Haemoglobin H Disease | 61/63 (96.8) 2/63 (3.2) |
| Body Mass Index1 | 23.8 [23.0, 24.6] |
| Current iron chelator2 Deferasirox Desferrioxamine | 56/63 (88.9) 7/63 (11.1) |
| Liver function tests | |
| Alanine transaminase (ALT)3(U/L) | 33.6 [27.2, 41.4] |
| Aspartate Aminotransferase (AST)3 (U/L) | 19.7 [16.1, 24.0] |
| Gamma-glutamyltranspeptidase (GGT)3(U/L) | 16.9 [14.3, 19.9] |
| Bilirubin1(umol/L) | 29.4 [27.4, 31.5] |
| International Normalised Ratio (INR) | 1.09 [1.02, 1.16] |
| Platelet Count (109/L) | 338.8 [300.7, 377.0] |
| Ferritin 1998 (N=60) (ng/mL)3 | 734.5 [601.6, 896.7] |
| Ferritin 2003 (N=61) (ng/mL)3 | 1041.9 [824.6, 1316.3] |
| Ferritin 2008 (N=63) ng/mL)3 | 1091.1 [908.8, 1310.0] |
| Ferritin 2013 (ng/mL)3 | 776.5 [633.9, 951.2] |
| Hepatic T2* (ms)3 | 7.6 [6.0, 9.6] |
| Hepatitis C virus IgG positive | 34/63 (54.0) |
| Hepatitis C viral load detectable | 17/63 (27.0) |
| Hepatitis C viral load (if detectable) (IU/mL)3 | 206097.6 [58044.8, 731783.9] |
| Hepatitis B virus surface antigen positive | 0/63 (0.0) |
| Hepatitis B virus core IgG positive | 7/63 (11.1) |
| Liver Stiffness Measurement (LSM) 3 | 6.8 [6.0, 7.7] |
| Enhanced Liver Fibrosis (ELF) Score1 | 9.5 [9.2, 9.7] |
Table 1: Baseline clinical and laboratory characteristics.Geometric mean (subsequent analysis performed on log-transformed data)
Figure 2: A: Ferritin concentration 1998-2013. Ferritin concentrations in the cohort increased between 1998 and 2003, remained stable until 2008, and then reduced to 2013.
B: ELF score and liver stiffness measurement
correlation. Non-invasive indices of hepatic fibrosis were closely correlated (r=0.473, p=0.0001).
LSM and ELF, Prevalence of Fibrosis
By ultrasound, only 1/63 patients demonstrated evidence of liver cirrhosis. By LSM, 18/63 (29%) had significant fibrosis (LSM >7.9kPa) including 6 (9.5%) with cirrhosis (LSM>13kPa). Two patients’ readings were invalid due to body habitus. By ELF, 34.4% of patients had evidence of severe fibrosis (ELF≥9.8). LSM and ELF score were correlated (r=0.47, P=0.0001) (Figure 2b). ELF scores had an AUCROC of 0.81 [95% CI 0.67, 0.95] to identify cirrhosis as identified by a LSM score >13.0kPa. An ELF score above 9.5 (50.8% of patients) had 100% sensitivity and 54.5% specificity for identifying cirrhosis.
Risk Factors for Fibrosis
Table 2 represents associations between independent variables and indices of fibrosis (LSM and ELF score). Age was associated with fibrosis risk by both LSM (P=0.0036) and ELF score (P=0.001). LSM was associated with present and historic ferritin: 1998 (P=0.002), 2003 (P=0.011), 2008 (P=0.026) and 2013 (P<0.001). Conversely, ELF score was not associated with ferritin. Both fibrosis measures were associated with indices of liver impairment and inflammation (ALT, AST, gGT, bilirubin and International Normalised Ratio (INR)) but neither was associated with platelet count, perhaps attributable to the high proportion of patients whom had undergone splenectomy (35/63). BMI does did not affect fibrosis in this population likely due to the low mean BMI (23.8 kg/m2). Presence of viral load positivity (active HCV) was a clear risk factor (P=0.006) for fibrosis by both measures. Interestingly, there was no correlation between liver (or cardiac) iron as measured by MRI T2* with fibrosis by either measure.
| LSM1 Coefficient [95% CI], P, N | ELF score Coefficient [95% CI], P | |
|---|---|---|
| Sex (M=1, F=0) | 0.06 [-0.04, 0.16], P=0.245 N=63 | 0.42 [-0.08, 0.91] P=0.096 N=61 |
| Age (years) | 0.0066 [0.0004, 0.0127], P=0.036 N=63 | 0.04 [0.01, 0.07] P=0.010 N=61 |
| Body Mass Index | 0.005 [-0.011, 0.021], P=0.504 N=63 | -0.03 [-0.10, 0.05] P=0.488 . N=61 |
| Liver function tests | ||
| Alanine transaminase (ALT)1 | 0.29 [0.17, 0.42], P<0.001 N=63 | 1.17 [0.51, 1.83] P=0.001 N=61 |
| Aspartate Aminotransferase (AST)1 | 0.37 [0.24, 0.50], P<0.001 N=61 | 1.46 [0.78, 2.15] P<0.001 N=61 |
| Gamma-glutamyltranspeptidase (GGT)1 | 0.34 [0.18, 0.50] P<0.001 N=63 | 0.99 [0.11, 1.87] P=0.027 N=61 |
| Bilirubin | 0.006 [0.0001, 0.013] P=0.046 N=63 | 0.02 [-0.01, 0.05] P=0.129 N=61 |
| International Normalised Ratio (INR) | 0.26 [0.06, 0.45] P=0.011 N=61 | 1.26 [0.35, 2.12] P=0.007 N=59 |
| Platelet Count (109/L) | -0.000 [-0.000, 0.001], P=0.192 N=63 | 0.000 [-0.001, 0.002] P=0.861 N=61 |
| Ferritin 1994 (n=32)1 | 0.26 [0.02, 0.50], P=0.037 N=32 | 0.71 [-0.28, 1.70] P=0.153 N=30 |
| Ferritin 1998 (n=60)1 | 0.25 [0.10, 0.40] P=0.002 N=60 | 1.14 [0.47, 1.81] P=0.001 N=58 |
| Ferritin 2003 (n=61)1 | 0.17 [0.04, 0.30] P=0.011 N=61 | 0.60 [-0.02, 1.21] P=0.057 N=59 |
| Ferritin 2008 (n=63)1 | 0.18 [0.02, 0.35] P=0.026 N=63 | 0.71 [-0.10, 1.51], P=0.083 N=61 |
| Ferritin 20131 | 0.26 [0.12, 0.40], P<0.001 N=63 | 0.38 [-0.40, 1.17] P=0.332 N=61 |
| Hepatic T2* (ms)1 | -0.12 [-0.25, 0.02], P=0.091 N=60 | -0.20 [-0.91, 0.51] P=0.571 N=58 |
| Cardiac T2* (ms)1 | -0.02 [-0.27, 0.22], P=0.844 N=60 | -0.28 [-1.72, 1..17] P=0.698 N=58 |
| Hepatitis C virus IgG positive (positive=1, negative=0) | 0.08 [-0.02, 0.19] P=0.118 N=63 | 0.04 [-0.47, 0.55] P=0.878 N=61 |
| Hepatitis C viral load detectable | 0.16 [0.05, 0.27] P=0.006 N=63 | 0.58 [0.04, 1.12] P=0.036 N=61 |
| Hepatitis C viral load (if detectable)1 | -0.03 [-0.15, 0.10] P=0.665 N=17 | 0.13 [-0.32, 0.58] P=0.555 N=17 |
Table 2: Associations between predictive variables and indices of fibrosis by univariate linear regression.
We attempted to control for confounding using multiple linear regression (Table 3). Age was associated with fibrosis risk using both ELF and LSM scores: ELF score was also associated with historic (1998) ferritin concentrations, while LSM score was also associated with presence of HCV persistent infection and both current (2013) and historic (1998) ferritin.
| N=60 Adj R2=0.4571 | LSM1 Coefficient [95% CI], P |
| Ferritin 1998 | 0.16 [0.03, 0.29] P=0.002 |
| Ferritin 2013 | 0.27 [0.145, 0.39] P<0.001 |
| Hepatitis C virus detectable | 0.15 [0.06, 0.24] P=0.002 |
| Age | 0.008 [0.003, 0.013] P=0.002 |
| N=58 Adj R2=0.2591 | ELF Score Coefficient [95% CI], P |
| Age | 0.04 [0.01, 0.07] P=0.005 |
| Ferritin 1998 | 1.27 [0.64, 1.91] P<0.001 |
Table 3: Independent associations between predictive variables and indices of fibrosis by multiple regression.
Prediction of Cirrhosis
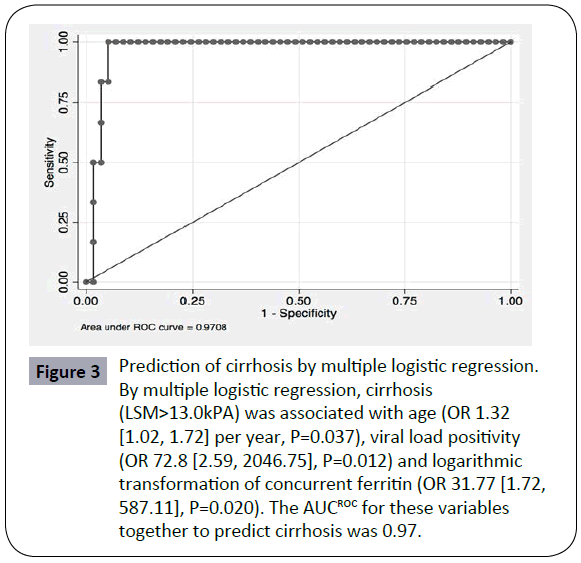
Using variables associated with LSM scores, we evaluated potential independent variables associated with cirrhosis (LSM>13.0KPa). Results of univariate logistic regression for variables associated with cirrhosis are shown in Supplementary Table 1: significant associations were found with age, ALT and viral load positivity. By multiple logistic regression, cirrhosis was associated with age (OR 1.32 [1.02, 1.72] per year, P=0.037), viral load positivity (OR 72.8 [2.59, 2046.75], P=0.012) and logarithmic transformation of concurrent ferritin (OR 31.77 [1.72, 587.11], P=0.020). The AUCROC for these variables together to predict cirrhosis was 0.97 (Figure 3). Of the six cirrhotic patients for whom complete data were available, all were aged 46 years or older, five of 6 had positive viral loads, and four had concurrent ferritin concentrations exceeding 500 ug/L.
Figure 3: Prediction of cirrhosis by multiple logistic regression.
By multiple logistic regression, cirrhosis (LSM>13.0kPA) was associated with age (OR 1.32 [1.02, 1.72] per year, P=0.037), viral load positivity (OR 72.8 [2.59, 2046.75], P=0.012) and logarithmic transformation of concurrent ferritin (OR 31.77 [1.72, 587.11], P=0.020). The AUCROC for these variables together to predict cirrhosis was 0.97.
Relationship between LSM and ELF Scores
LSM and ELF gave concordant findings (cirrhosis for LSM, ELF for severe fibrosis) in 5/6 patients with cirrhosis and 39/55 without cirrhosis. Levels of ALT and AST were higher and HCV viral load was more often detectable in discordant individuals, indicating that discordance between the two indices could be potentially related to the effect of liver inflammation in the setting of active HCV which can confound assessment of liver stiffness [28,29] (Table 2).
Discussion
In this cohort of patients with transfusion dependent thalassaemia, 9.5% had unsuspected cirrhosis, 29% had unexpected fibrosis by LSM and 34.4% had evidence of severe fibrosis by ELF scores. Risk factors for fibrosis included age, contemporary high ferritin concentration, active HCV and historical ferritin. In recent years, ferritin levels have fallen, perhaps due to oral iron chelation. However, our data suggest that the consequences of previous poor control are likely to impact the future burden of disease. The high prevalence of fibrosis supports a practice of screening for cirrhosis and associated complications including regular surveillance for HCC in all adults with TH.
LSM has been demonstrated to reliably identify advanced fibrosis in patients with thalassaemia in three previous studies [12,13,30]. Mirault et al. compared LSM to biopsy in 15 chronically transfused patients: LSM values above 6.25 kPa identified patients at risk for severe fibrosis (sensitivity 80%, specificity 70%, negative predictive value 88%; positive predictive value 57%) [12]. Di Marco et al. compared LSM with biopsy in 56 thalassaemic patients prior to anti-HCV therapy or splenectomy; LSM had an excellent AUCROC for cirrhosis (0.997) and a cut-off of 13Kpa had 100% and 95% sensitivity and specificity respectively [13]. Fraquelli et al. studied LSM in 115 patients, with equal proportion of transfusion dependent thalassaemia and thalassaemia intermedia and found that LSM was associated with ALT, gGT, bilirubin and HCV RNA viral load, but not with ferritin. LSM was validated in a subset of 14 patients and confirmed the approach was appropriate. According to the TE results, significant fibrosis (LSM > 7.9 kPa), severe fibrosis (LSM >10.3 kPA) and cirrhosis (LSM >12 kPA) was present in 35%, 24% and 17% respectively [30]. Collectively, these studies indicate that non-invasive assessment for fibrosis in patients with thalassaemia is reliable, and confirm our finding that fibrosis is highly prevalent in this population. ELF score correlates closely with LSM scores in β-thalassaemia and can be used to predict the presence of cirrhosis. In settings where TE is unavailable, the ELF panel may provide similar information, although further validation against liver biopsy in this context is needed. In a recent study the addition of serum markers to LSM has been shown to increase the accuracy of fibrosis detection and this may present another role for ELF measurement in this population [24]. International guidelines now support routine LSM in thalassaemic patients with HCV; our data suggest this should be extended to adult thalassaemic patients with a history of current or historic of iron loading [5].
Iron loading by T2* MRI did not correlate with liver fibrosis by either LSM or ELF score as reported by both Mirault et al. and Fraquelli et al. [12,30]. However, we found that both current and historic serum ferritin correlated with liver fibrosis, indicating that liver damage is inflicted by sustained rather than contemporaneous iron loading. The potential mechanism of hepatic damage induced by siderosis includes alterations of the hepatocyte membrane, intranuclear cytoplasmic pseudoinclusions, swollen mitochondria and variations in the endoplasmic reticuli leading to ballooning of hepatocytes and focal necrosis [23]. Chronic HCV infection may independently exacerbate iron overload, potentially through direct suppression of hepcidin expression and enhanced iron absorption [31]. Necro-inflammation associated with HCV can also cause biochemical and tissue iron excess [32,33]. The excess iron promotes the formation of free radicals and increases oxidative stress leading to the development of fibrosis [34]. Our data confirms for the first time the importance of both antecedent and concurrent iron loading as a risk factor for hepatic fibrosis.
We identified independent effects on fibrosis risk of iron loading and active HCV. In a study of thalassaemia patients post bone marrow transplant, hepatic iron loading and HCV predicted progression of liver fibrosis with the combination having a synergistic effect [35]. A study of 95 patients with HCV undergoing liver biopsy identified hepatic iron overload in 31.6%, which was associated with a more severe stage of liver fibrosis [36]. The potential of an increased risk of fibrosis progression due to dual risk factors and thereby the development of cirrhosis need to be considered. A multicentre survey of Italian thalassaemia centres found that HCC is becoming more frequent as these patients survive into older age [4]. A prospective study conducted on 108 thalassaemia patients (38 with TM and 70 with TI; median age 36.8 years) estimated an HCC incidence of about 2% [37].
Improvement in iron overload management has lead to increased survival of thalassaemic patients, chiefly due to improved cardiac iron chelation [38,39]. The risk factors of age, duration of disease, persistent viraemia and iron overload increase fibrosis progression and the future burden of liver disease in this group. Our data emphasise the importance of treating HCV and iron overload aggressively to minimise further damage. The introduction of novel HCV treatments, which are interferon and ribavirin-free and hence offset the need for increased blood transfusion, are encouraging [40]. These are associated with high rates of virological response in the general population and we are evaluating treatment efficacy and tolerability in thalassaemia patients at our institution.
There are a few limitations to our study, the most significant of which is that it is retrospective. Furthermore, there was no correlation with liver biopsy. Liver biopsy is invasive and there is poor uptake with asymptomatic individuals. At our institution, patients with hepatitis C are screened for fibrosis almost exclusively with LSM.
The presence of HCV and current and historical iron loading have an additive effect in fibrosis risk in patients with transfusion dependent thalassaemia. This population is at risk for the development of cirrhosis and HCC associated with significant future disease burden. We recommend all adult patients; particularly those with present or historical iron overload, abnormal biochemical markers and HCV should undergo assessment for fibrosis using LSM or similar methods where available. We have instituted this approach in our centre. Where non-invasive methods are unavailable, routine screening liver biopsies to assess for cirrhosis should be considered.
References
- Prati D, Maggioni M, Milani S, Cerino M, Cianciulli P, Coggi G, et al. (2004) Clinical and histological characterization of liver disease in patients with transfusion-dependent beta-thalassaemia. A multicenter study of 117 cases. Haematologica 89: 1179-1186.
- Mancuso A (2010) Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol 2: 171-174.
- Olivieri NF (1999) The beta-thalassemias. N Engl J Med 341: 99-109.
- Borgna-Pignatti C, Vergine G, Lombardo T, Cappellini MD, Cianciulli P, et al. (2004) Hepatocellular carcinoma in the thalassaemia syndromes. Br J Haematol 124: 114-117.
- Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V (2014) Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT) [Internet]. Nicosia, Cyprus, Thalassaemia International Federation.
- Choong CC, Venkatesh SK, Siew EP (2012) Accuracy of routine clinical ultrasound for staging of liver fibrosis. J Clin Imaging Sci 2: 58.
- Piccinino F, Sagnelli E, Pasquale G, Giusti G (1986) Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 2: 165-173.
- Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, et al. (2007) Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 14: 360-369.
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, et al. (2006) Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 55: 403-408.
- Castera L, Forns X, Alberti A (2008) Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 48: 835-847.
- Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, et al. (2008) Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 134: 960-974.
- Mirault T, Lucidarme D, Turlin B, Vandevenne P, Gosset P, et al. (2008) Non-invasive assessment of liver fibrosis by transient elastography in post transfusional iron overload. Eur J Haematol 80: 337-340.
- Di Marco V, Bronte F, Cabibi D, Calvaruso V, Alaimo G, et al. (2010) Noninvasive assessment of liver fibrosis in thalassaemia major patients by transient elastography (TE) - lack of interference by iron deposition. Br J Haematol 148: 476-479.
- Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, et al. (2004) Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 127: 1704-1713.
- Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, et al. (2013) The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 59: 236-242.
- Parkes J, Guha IN, Roderick P, Rosenberg W (2006) Performance of serum marker panels for liver fibrosis in chronic hepatitis C. J Hepatol 44: 462-474.
- Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, et al. (2007) Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology47:455-460.
- Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, et al. (2008) Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology 48: 1549-1557.
- Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, et al. (2009) Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 136: 160-167.
- Parkes J, Roderick P, Harris S, Day C, Mutimer D, et al. (2010) Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59: 1245-1251.
- Crespo G, Fernández-Varo G, Mariño Z, Casals G, Miquel R, et al. (2012) ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol 57: 281-287.
- Pinzani M (2010) The ELF panel: a new crystal ball in hepatology? Gut 59: 1165-1167.
- Thakerngpol K, Fucharoen S, Boonyaphipat P, Srisook K, Sahaphong S, et al. (1996) Liver injury due to iron overload in thalassemia: histopathologic and ultrastructural studies. Biometals 9: 177-183.
- Boursier J, de Ledinghen V, Zarski JP, Rousselet MC, Sturm N, et al. (2011) A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am J Gastroenterol 106: 1255-1263.
- Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, et al. (2006) Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J GastroenterolHepatol 18: 411-412.
- Mavrogeni SI, Gotsis ED, Markussis V, Tsekos N, Politis C, et al. (1998) T2 relaxation time study of iron overload in b-thalassemia. MAGMA 6: 7-12.
- Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, et al. (2001) Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 22: 2171-2179.
- Tapper EB, Cohen EB, Patel K, Bacon B, Gordon S, et al. (2012) Levels of Alanine Aminotransferase Confound Use of Transient Elastography to Diagnose Fibrosis in Patients With Chronic Hepatitis C Virus Infection. Clinical Gastroenterology and Hepatology 10:932-937.
- Sagir A, Erhardt A, Schmitt M, Häussinger D (2008) Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 47: 592-595.
- Fraquelli M, Cassinerio E, Roghi A, Rigamonti C, Casazza G, et al. (2010) Transient elastography in the assessment of liver fibrosis in adult thalassemia patients. Am J Hematol 85: 564-568.
- Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA (2008) Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology48:1420-1429.
- Bonkovsky HL, Banner BF, Rothman AL (1997) Iron and chronic viral hepatitis. Hepatology 25: 759-768.
- Pietrangelo A (2003) Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology 124: 1509-1523.
- Philippe MA, Ruddell RG, Ramm GA (2007) Role of iron in hepatic fibrosis: one piece in the puzzle. World J Gastroenterol 13: 4746-4754.
- Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, et al. (2002) Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood 100: 17-21.
- Souza RM, Freitas LA, Lyra AC, Moraes CF, Braga EL, et al. (2006) Effect of iron overload on the severity of liver histologic alterations and on the response to interferon and ribavirin therapy of patients with hepatitis C infection. Braz J Med Biol Res 39: 79-83.
- Mancuso A, Sciarrino E, Renda MC, Maggio A (2006) A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin 30: 119-124.
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, et al. (1994) Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 331: 574-578.
- Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, et al. (2004) Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 89: 1187-1193.
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, et al. (2014)Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences