Analysis of Adherence to AllOral HCV Therapy in a Cohort of People who Inject Drugs (PWID)
Rajvir Shahi, Ghazaleh Kiani, Arshia Alimohammadi, Tyler Raycraft, Arpreet Singh and Brian Conway
DOI10.21767/2471-9706.100017
Rajvir Shahi, Ghazaleh Kiani, Arshia Alimohammadi, Tyler Raycraft, Arpreet Singh and Brian Conway*
Vancouver Infectious Diseases Centre, 201-1200 Burrard St., Vancouver, BC, Canada
- *Corresponding Author:
- Brian Conway
Vancouver Infectious Diseases Centre
201- 1200 Burrard St., Vancouver, BC, Canada.
Tel: 6046426429
E-mail: Brian.Conway@vidc.ca
Received date: June 24, 2016; Accepted date: January 04, 2017; Published date: January 12, 2017
Citation: Shahi R, Kiani G, Alimohammadi A, et al. Analysis of Adherence to All-Oral HCV Therapy in a Cohort of People who Inject Drugs (PWID). J Hep. Vol. 3 No. 1: 1. doi: 10.21767/2471-9706.100017
Abstract
The majority of prevalent and incident cases of HCV infection in Canada occur among people who inject drugs (PWID). The benefits of more effective and better tolerated all-oral regimens will only be achieved with proper strategies to ensure adherence in this population. We hypothesize that the provision of treatment within a multidisciplinary centre attending to all health care needs will maximize the likelihood of achieving this goal. Within a cohort of 87 patients receiving all oral HCV therapy over a median of 12 weeks, 1135/1150 (98.7%) scheduled weekly appointments were attended, with pill counts reflecting over 90% adherence to therapy. In this model of care, adherence to HCV therapy among PWID met or exceeded the standard established in clinical trials. These data directly address one of the major concerns about increasing availability of HCV treatment among PWID and lend further support to the expansion of such programs in clinical practice.
Keywords
HCV therapy; Drugs; Health care; Antiretroviral
Introduction
The high prevalence of hepatitis C virus (HCV) infection is a serious worldwide public health concern. People who inject drugs (PWID) account for a substantial portion of new (80%) and existing (60%) cases of HCV in developed countries, including Canada [1,2]. Needle syringe programs (NSP) and opioid substitution treatment (OST) have had limited success in preventing HCV [3-5]. Although the recent development of interferon-free direct acting antiretroviral (DAA)-based therapies and all- oral regimens have shown to produce higher sustained virologic response (SVR) rates (90% or higher) with few if any side effects, successful treatment is also dependent on adherence to prescribed therapy [6]. This has traditionally been considered a challenge in PWID [6].
Previous Canadian guidelines required at least 6 months of abstinence from drug use before the initiation of treatment due to concerns about poor adherence as well as the risk of recurrent viremia after a successful course of therapy [7]. Current guidelines suggest that the initiation of treatment without the abstinence period be considered in the context of a multidisciplinary care program, that could address these concerns [8,9]. Despite these new guidelines, it appears that only 20% of Canadian HCV specialists are willing to provide treatment to HCV-infected individuals who are concurrently injecting recreational drugs [10].
There is limited evidence to confirm that the proposed care delivery model will address the concerns that have, to date, limited access of PWID to HCV therapy. Although data on recurrent viremia will need to be generated over several years, information about adherence can readily be ascertained as programs are initiated. We report on adherence in a program designed to provide HCV therapy to PWID within a supportive, multidisciplinary context. The total number of HCV-positive, PWID followed at our multidisciplinary center is 239 individuals, with 115/239 (48.1%) of these PWID currently receiving treatment at our centre. Analysis of adherence to all-oral HCV therapy was completed for 87 subjects.
Methods
Adherence data were generated among PWID receiving alloral HCV treatment between April/2015-March/2016 at a multidisciplinary clinic in Vancouver. In addition to HCV therapy, our centre provides services to address relevant medical, social and psychologic concerns. HCV treatment regimens were chosen according to contemporary clinical guidelines (duration 12-24 weeks), with weekly follow-up scheduled to dispense medications and update plans of care. Adherence was measured in 2 ways: attendance at scheduled clinic visits, expressed as a proportion of visits for which attendance was positively documented ( ± 3 days); pill counts, performed at each visit, as a confirmatory measure, with consumption of over 90% prescribed medications in the last interval allowing us to count the clinic visit as a true measure of patient adherence. The inclusion criteria for this trial of PWID: Individuals who were documented to have HCV and who had actively been injecting drugs within 6-9 months of treatment initiation. Subjects selected were male or female and 19 years or older, and on all-oral HCV treatment regimens for a duration of 12 to 24 weeks. Active drug use was assessed through patient questionnaires and interviews, and via urine drug screens.
Results
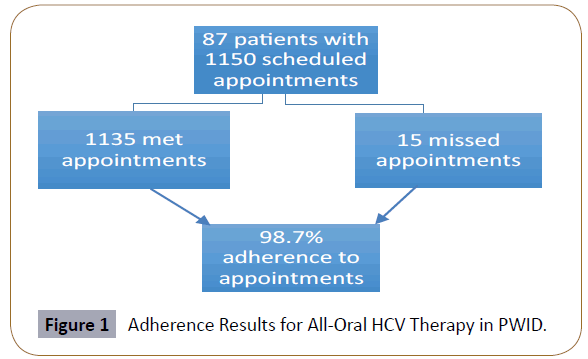
Analysis of adherence to all-oral HCV therapy has been completed for 87 subjects. Key demographic characteristics are shown in Table 1. The mean age was 55 (Range 34-75), 65% were male, 29% were opioid users, 18% were cocaine users, 18% were stimulant users, 29% were on methadone substitution therapy, and 82% were on a 12-week regimen of HCV medications (with 18% receiving longer courses of treatment, up to 24 weeks, based on disease state or prior treatment history). Of the 87 subjects, 32% had psychiatric comorbidities, 39% used alcohol, 19% had unstable housing, 14% were on welfare or disability, 28% were working or receiving pension, and 23% were unemployed. As shown in Figure 1, adherence to total of 1150 scheduled appointments was 98.7% (1135/1150 scheduled appointments were met; 15 appointments were missed). Pill counts reflected over 90% adherence to therapy at all clinic visits, allowing us to validate clinic attendance as a marker of adherence, at least in this context.
| Characteristics of Study Participants | |
|---|---|
| Mean Age (years) | 55 (Range 34-75) |
| Male (n, %) | 57 (65%) |
| Opioid Users (n, %) | 25 (29%) |
| Cocaine Users (n, %) | 16 (18%) |
| Stimulant Users (%) | 16 (18%) |
| Methadone Substitution Therapy (%) | 25 (29%) |
| 12-Week Regimen of HCV Therapy (%) | 71 (82%) |
| Psychiatric Comorbidities | 28 (32%) |
| Alcohol Use | 34 (39%) |
| Unstable Housing | 17 (19%) |
| Welfare or Disability | 12 (14%) |
| Working or Receiving Pension | 24 (28%) |
| Unemployed | 20 (23%) |
Table 1: Demographic characteristics of study participants (N=87).
Discussion
Until recently, HCV treatment of active PWID was often withheld by physicians due to concerns about poor adherence, factors associated with concurrent substance use, the risk of reinfection after successful therapy, and comorbid psychiatric diseases that could not be properly addressed [11]. Missed appointments and lack of adherence to HCV treatment regimens are especially problematic in these patients, due to the sociodemographic and cognitive-affective factors that are often present in this population [12,13].
To address these concerns, we provided HCV-infected PWID with treatment within a multidisciplinary model of health care delivery. Our team includes the primary care provider - an infectious disease specialist - nurses, clinical researchers, and access to addiction and social support services, including a weekly HCV Support Group. With adherence to scheduled appointments at 98.7% and pill counts reflecting greater than 90% adherence, our data indicate that when services to address the medical, social, and psychologic needs of the patient are provided, individuals are more likely to benefit from HCV treatment. In our study, subjects included had a variety of sociodemographic and cognitive-affective factors present. Treatment with all-oral HCV regimens in a multidisciplinary centre addressing all of their health care needs was likely responsible for the high adherence rates in this cohort. It is particularly encouraging to see such high adherence levels in PWID, at least as represented in our clinic population (largely ongoing mixed opiate and stimulant use, with a minority on opiate substitution therapy). Going forward, it will be important to determine if the level of adherence we have documented will translate into the expected level of efficacy and that long-term engagement in care post-SVR will help reduce the rate of recurrent viremia.
Conclusion
In conclusion, we demonstrate that high adherence to HCV therapy among PWID can be achieved in the context of a multidisciplinary program of care. We believe this addresses one of the major concerns about increasing availability of HCV treatment in this population and lends further support to the expansion of such programs in clinical practice.
References
- Shepard CW, Finelli L (2005) Global epidemiology of hepatitis C virus infection. Alter Med J 20: 12-19.
- Palella FJ, Delaney KM, Moorman AC (1997) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. Lancet Infect Dis 5: 558-567.
- Nelson PK, Mathers BM, Cowie B (2011) Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 378: 571-583.
- Hagan H, Pouget ER, Des Jarlais DC (2011) A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis 204: 74-83.
- Sacks-Davis R, Horyniak D, Grebely J, Hellard M (2012) Behavioural interventions for preventing hepatitis C infection in people who inject drugs: A global systematic review. Int J Drug Policy 23: 176-184.
- Turner KM, Hutchinson S, Vickerman P (2011) The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction 106: 1978-1988.
- Hagan LM, Yang Z, Ehteshami M, Schinazi RF (2013) All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat 20: 847-857.
- Sherman M, Shafran S, Burak K (2007) Management of chronic hepatitis C: consensus guidelines. Canadian Journal of Gastroenterology 21: 25C-34C.
- Myers R, Shah H, Burak K, Cooper C, Feld J (2015) An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Can J Gastroenterology Hepatology 29: 19-34.
- Chung R, Davis G, Jensen D (2015) Hepatitis C guidance: AASLDÃÆâÃâââ¬ÃâÃÂIDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 62: 932-954.
- Myles A, Mugford GJ, Zhao J, Krahn M, Wang PP (2011) Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: results from a national specialist survey in Canada. Can J Gastroenterol 25: 135-139.
- Grebely J, Tyndall MW (2011) Management of HCV and HIV infections among people who inject drugs. Curr Opin HIV AIDS 6: 501-507.
- Swan D, Long J, Carr O (2010) Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 24: 753-762.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences